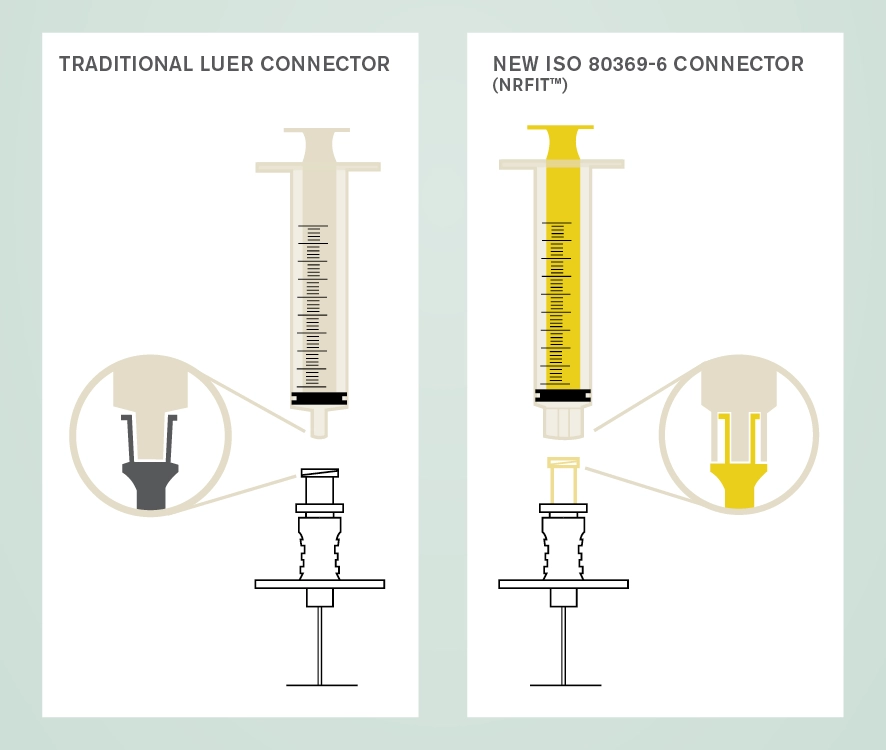
The new design is similar to Luer with a slightly smaller mating interface. A trademark name associated with devices compliant with this standard is NRFit®. There are three main suppliers of neural connectors in Australia; B-Braun, Pajunk and Smiths. A key role of procurement officers will be in ensuring smooth transition to the use of these new connectors.
Why is this change happening?
The International Organization for Standardization (ISO) has overseen the development of a series of connectors for devices used for different clinical applications as a safety initiative to avoid wrong route administration of medicines and substances.
The ISO 80369 series of standards (known as Small Bore Connectors) define the dimensions of the connectors for different clinical uses which cannot couple with each other. Part 6 of this series pertains to connectors used with devices for neuraxial and other neural procedures and is known as ISO 80369-6 Small bore connectors for liquids and gases in healthcare applications — Part 6: Connectors for neuraxial applications.
Related resources
We've collated a comprehensive collection of ACSQHC advisories, articles, publications, and case studies relating to the changeover from Luer devices to ISO 80369-6 devices in our NRFit Changeover Library Guide, including a fact sheet for practitioners.
Explore our other standards of practice
Our professional documents (prof docs), statements and guidelines are crucial for promoting the safety and quality of patient care for those undergoing anaesthesia for surgical and other procedures and for those receiving pain medicine treatment.
We've contributed to or endorsed a selection of guidelines and statements developed by other organisations.
These statements undergo a deliberative process within the college committee structure and require approval by the oversight committee, or the ANZCA Council, prior to publication.
The Standards for Anaesthesia and its accompanying background paper are intended to apply to all those in clinical practice within the specialty of anaesthesia wherever anaesthesia services are provided. The aim is for these standards to be used as benchmarks to foster quality in patient care as well as to facilitate quality improvement.
We're responsible for setting the standards of clinical practice for pain medicine in Australia and New Zealand. Our activities in this area include developing professional documents, issuing safety alerts; and overseeing incident reporting activities.
The Standards for Perioperative Medicine have been released for a six-month pilot period ending in June 2024. These standards are intended to apply to all medical practitioners whose practice falls within the scope of perioperative medicine. The purpose of these standards is to articulate standards and associated indicators of quality care against which performance can be compared.
We oversee a range of incident reporting activities in Australia and New Zealand, including mortality reporting and webAIRS, to ensure that we continue to be two of the safest countries in the world to undergo anaesthesia.
These management and diagnosis cards have been jointly produced by ANZCA and the Australian and New Zealand Anaesthetic Allergy Group (ANZAAG). They are designed to be used as a crisis management package in the event of an acute perioperative anaphylaxis.
The Perioperative Care Framework was developed by the Perioperative Care Working Group approved by ANZCA Council in December 2021, and updated in December 2023.
To assist fellows and trainees, the college has also developed a framework for understanding professionalism and performance as it applies to the practice of anaesthesia and pain medicine: Supporting professionalism and performance – A guide for anaesthetists and pain medicine physicians.
